Research Overview—
The Wolfe lab studies intramembrane-cleaving proteases (I-CLiPs) that play critical roles both in normal biology and in human disease. The last place in the cell to expect hydrolysis is within the hydrophobic environment of the lipid bilayer. Nevertheless, a number of multi-pass membrane proteins appear to carry out this seemingly paradoxical process (see the figure). Such proteases cut within the transmembrane region of their respective substrates, and consistent with this observation, these proteases contain putative catalytic residues located within transmembrane domains.
The major focus of the lab has been on the chemistry and biology of γ-secretase. This protease is critical to the pathogenesis of Alzheimer's disease and to cell differentiation during embryonic development and adulthood. The enzyme is responsible for producing the amyloid β-peptide (Aβ) that deposits in the Alzheimer brain and is considered an important target for the development of potential therapeutics. The protease also cleaves Notch proteins as an essential part of the signaling mechanism of these receptors that regulate cell-fate determination. The Wolfe lab developed transition-state analogue inhibitors and used these as tools to characterize and identify γ-secretase. Findings from the lab implicated a membrane protein called presenilin as the catalytic component of a large γ-secretase complex. Missense mutations in presenilin cause hereditary Alzheimer's disease, and these mutations specifically affect γ-secretase activity. In the process of purifying γ-secretase, we found that an endogenous substrate also copurified, suggesting an initial substrate docking site on the protease complex distinct from the active site. Helical peptides designed to interact with this docking site can potently inhibit γ-secretase activity. The enzyme also has two distinct proteolytic functions: an endopeptidase activity and a carboxypeptidase activity that trims initially formed long Aβ peptides to shorter secreted forms. Alzheimer-causing mutations in presenilin dramatically decrease the carboxypeptidase activity of γ-secretase, leading to increased proportions of long Aβ peptides. Current efforts are aimed at further elucidating the mechanism of γ-secretase substrate recognition and its multiple proteolytic functions, understanding how genetic mutations that cause Alzheimer’s disease alter γ-secretase structure and function, and developing new chemical probes and therapeutic prototypes targeting this protease complex.
The Wolfe lab has also investigated the structure, mechanism, and inhibition of other intramembrane proteases, such as the serine protease Rhomboid and the presenilin homolog signal peptide peptidase, both of which are conserved across evolution and play critical roles in biology. In this way, the lab helped establish common biochemical principles and strategies for designing inhibitors for this family of membrane-embedded enzymes. The current focus is developing new chemical probes to understand the roles of these membrane-embedded proteases in biology, particularly in human parasites such as Plasmodium falciparum that causes malaria, and explore the potential of these proteases as therapeutic targets.
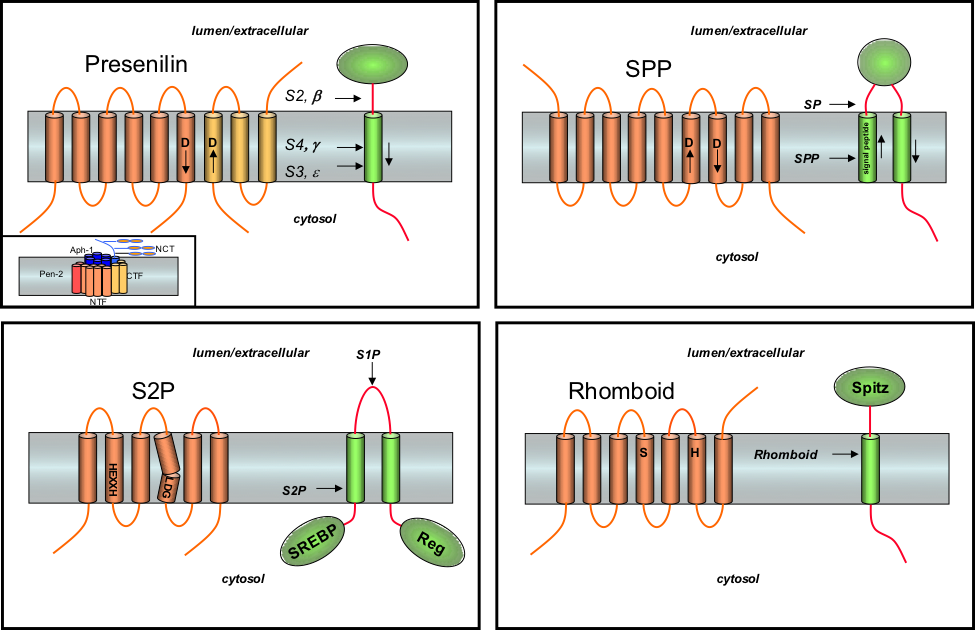 Representative I-CLiPs. Top left: Presenilin is the catalytic component of a large γ-secretase complex (inset), an aspartyl protease that cleaves within the transmembrane domain of the amyloid precursor protein, Notch receptors, and other type I integral membrane proteins. Top right: Signal peptide peptidase (SPP) is part of a family of presenilin homologs. These aspartyl proteases are active on their own and have inverted membrane topology relative to presenilins. SPP cleaves remnant signal peptides in the membrane produced by signal peptidase. Bottom left: the Site 2 Protease (S2P) family are metalloproteases, with the transmembrane motifs coordinating with zinc in the active site. The human S2P enzyme cleaves a transcription factor (SREBP) that regulates cholesterol biosynthesis. Bottom right: The Rhomboid family of serine proteases are found in virtually all forms of life and are involved in many biological processes. In Drosophila, Rhomboid cleaves a growth factor called Spitz. Figure taken from Wolfe and Kopan, Science, 2004.
Representative I-CLiPs. Top left: Presenilin is the catalytic component of a large γ-secretase complex (inset), an aspartyl protease that cleaves within the transmembrane domain of the amyloid precursor protein, Notch receptors, and other type I integral membrane proteins. Top right: Signal peptide peptidase (SPP) is part of a family of presenilin homologs. These aspartyl proteases are active on their own and have inverted membrane topology relative to presenilins. SPP cleaves remnant signal peptides in the membrane produced by signal peptidase. Bottom left: the Site 2 Protease (S2P) family are metalloproteases, with the transmembrane motifs coordinating with zinc in the active site. The human S2P enzyme cleaves a transcription factor (SREBP) that regulates cholesterol biosynthesis. Bottom right: The Rhomboid family of serine proteases are found in virtually all forms of life and are involved in many biological processes. In Drosophila, Rhomboid cleaves a growth factor called Spitz. Figure taken from Wolfe and Kopan, Science, 2004.
Selected Publications —
Research Reports
- Liu Y, Rodriguez L, Wolfe MS. Template-directed synthesis of a small molecule-antisense conjugate targeting an mRNA structure. Bioorg. Chem. 2014; 54:7-11.
- Holmes O, Paturi S, Wolfe MS, Selkoe DJ. Functional analysis and purification of a Pen-2 fusion protein for γ-secretase structural studies. J. Neurochem. 2014; 131(1):94-100
- Holmes O, Paturi S, Selkoe DJ, Wolfe MS. Pen-2 is essential for γ-secretase complex stability and trafficking but partially dispensable for endoproteolysis. Biochemistry. 2014; 53(27):4393-406.
- Fernandez MA, Klutkowski JA, Freret T, Wolfe MS. Alzheimer Presenilin-1 Mutations Dramatically Reduce Trimming of Long Amyloid β-Peptides (Aβ) by γ-Secretase to Increase 42-to-40-Residue Aβ. J Biol Chem 2014; 289(45):31043-52.
- Park HJ, Ran Y, Jung JI, Holmes O, Price AR, Smithson L, Ceballos-Diaz C, Han C, Wolfe MS, Daaka Y, Ryabinin AE, Kim SH, Hauger RL, Golde TE, Felsenstein KM. The stress response neuropeptide CRF increases amyloid-β production by regulating γ-secretase activity. EMBO J 2015; 34(12): 1674-86.
- Bolduc DM, Montagna DR, Gu Y, Selkoe DJ, Wolfe MS. Nicastrin functions to sterically hinder γ-secretase-substrate interactions driven by substrate transmembrane domain. Proc Natl Acad Sci USA 2016 113(5):E509-18.
- Lu D, Wei HX, Zhang J, Gu Y, Osenkowski P, Ye W, Selkoe DJ, Wolfe MS, Augelli-Szafran CE. Part 1: Notch-sparing γ-secretase inhibitors: The identification of novel naphthyl and benzofuranyl amide analogs. Bioorg Med Chem Lett 2016, 26(9):2129-32.
- Wei HX, Lu D, Sun V, Zhang J, Gu Y, Osenkowski P, Ye W, Selkoe DJ, Wolfe MS, Augelli-Szafran CE. Part 2. Notch-sparing γ-secretase inhibitors: The study of novel γ-amino naphthyl alcohols. Bioorg Med Chem Lett 2016, 26(9):2133-37.
- Zhang J, Lu D, Wei HX, Gu Y, Selkoe DJ, Wolfe MS, Augelli-Szafran CE. Part 3: Notch-sparing γ-secretase inhibitors: SAR studies of 2-substituted aminopyridopyrimidinones. Bioorg Med Chem Lett 2016, 26(9):2138-41.
- Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of γ-secretase. eLife 2016, DOI: http://dx.doi.org/10.7554/eLife.17578
- Fernandez MA, Biette K, Dolios G, Seth D, Wang R, Wolfe MS. Transmembrane substrate determinants for γ-secretase processing of APP CTF-β. Biochemistry 2016,55(40):5675-5688.
Reviews and Commentaries
- Wolfe MS. Targeting mRNA for Alzheimer’s and related dementias. Scientifica 2014. Article ID 757549
- Wolfe MS, Selkoe DJ. γ-Secretase: a horseshoe structure brings good luck. Cell. 2014;158(2):247-9.
- Bolduc DM, Wolfe MS. Structure of nicastrin unveils secrets of γ-secretase. Proc Natl Acad Sci U S A. 2014 Oct 14;111(41):14643-4.
- Wolfe MS. Aspartic proteases of Alzheimer’s disease: β- and γ-secretases. In: Ralph A Bradshaw and Philip D Stahl (Editors-in-Chief), Encyclopedia of Cell Biology, Vol 1, Waltham, MA: Academic Press, 2016, pp. 661-9.
- Wolfe MS. Cutting in on a secretase pas de deux. Cell Res 2015; 25(10):1091-2.
- Wolfe MS. Cutting to the chase: How pathogenic mutations cause Alzheimer’s disease. J Exp Med 2015; 212(12): 1991.
- Wolfe MS, Yankner BA. Sorting Out Presenilins in Alzheimer’s Disease. Cell 2016, 166(1):13-5.
Patents and Patent applications
- (Hydroxyethyl)ureas as inhibitors of Alzheimer’s beta-amyloid production.
Inventors: M. S. Wolfe and D. J. Selkoe.
U.S. Patent No. 6,696,488 - Helical Peptidomimetics
Inventor: M. S. Wolfe
U.S. Patent No. 6,846,805 - Helical Peptidomimetics with Enhanced Activity
Inventor: M. S. Wolfe
U.S. Patent No. 7,501,397

