Design and Synthesis of Proteinase Inhibitors
Many critical biological processes are initiated, sustained or terminated by the action of a proteinase or peptidase enzyme. Uncontrolled proteolysis is a significant factor in many pathophysiological processes, yet there are almost no drugs with which to address this. Our research has been directed at the design and evaluation of new types of inhibitors for proteinase enzymes.
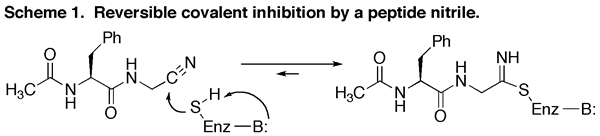
We were the first to demonstrate (by means of C-13 NMR) that the potent reversible inhibition of cysteine proteinases by peptide nitriles was due to their ability to form covalent thioimidate adducts (i.e. reactive intermediate analogs) leading to very potent but fully reversible inhibition.
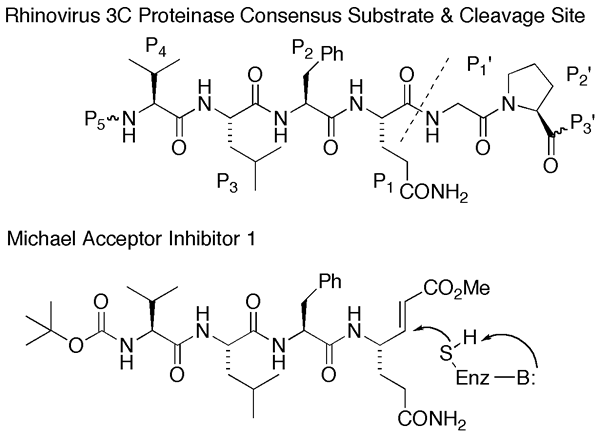
We also designed and synthesized the first peptidyl Michael acceptors and showed them to be extremely potent irreversible inactivators of cysteine proteases. Working with Professor Jeffrey Aubé in our department, we made Michael acceptor inhibitors for the 3C protease of human rhinovirus, which is the major cause of the common cold. Several of our compounds proved to be extremely potent inhibitors of the rhinovirus 3C protease and had sub-micromolar IC50 values for inhibition of viral replication in vitro.
Still more recent work has proceeded along two parallel lines, one is focused at developing more new chemistry for protease inhibition, and the other is focused at designing inhibitors for specific biologically relevant targets. Chief among the latter is calpain-I, a calcium-activated neutral protease found in cytosol of mammalian cells, which is thought to be involved in cytotoxic responses and degenerative diseases in nerve, heart and muscle tissue. Another important target is the 3C proteinase induced in mammalian cells infected with Coxsackie virus, a major cause of debilitating heart disease in humans.
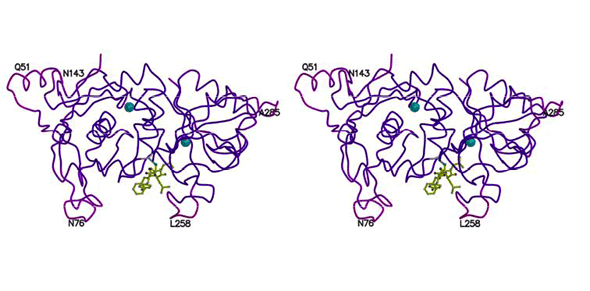
We recently have begun a major effort focused on the structure-based design of inhibitors for calpain-I. This has been a collaborative effort between our group and several other KU faculty members in Medicinal Chemistry (J. Aubé and E. Schönbrunn) and Molecular Biosciences (R. Weaver). We recently have determined the structure of the protease core of recombinant human calpain-I by X-ray crystallography. Another part of this project involves the synthesis of potential inhibitors using combinatorial chemistry and parallel synthesis approaches. In addition the KU High-Throughput Screening Laboratory will use our recombinant human calpain-I to screen the many thousands of compounds in their collections to discover new lead compounds.
Publications
- Qingshan Li, Robert P. Hanzlik, Robert F. Weaver, and Ernst Schönbrunn, "The Molecular Mode of Action of a Covalently Inhibiting Peptidomimetic on the Human Calpain Protease Core." Biochemistry, 45, 701-708 (2006).
- Y. Zeng, Q. Li, R. P. Hanzlik, and J. Aubé, "Synthesis of a Small Library of Diketopiperazines as Potential Inhibitors of Calpain." Bioorg. Med. Chem. Lett., 15, 3034-3038 (2005).
- S. Venkatraman, J.-S. Kong, S. Nimkar, Q. M. Wang, J. Aubé, and R. P. Hanzlik, "Design, Synthesis and Evaluation of Azapeptides as Substrates and Inhibitors for Human Rhinovirus 3C Protease." Bioorg. Med. Chem. Let., 9, 577-580 (1999).
- J.-S. Kong, S. Venkatraman, K. Furness, S. Nimkar, T. A. Shepherd, Q. M. Wang, J. Aubé, and R. P. Hanzlik, "Synthesis and Evaluation of Peptidyl Michael Acceptors That Inactivate Human Rhinovirus 3C Protease and Inhibit Virus Replication." J. Med. Chem. 41, 2579-2587 (1998).
- R. Xing and R. P. Hanzlik, "Azapeptides as Inhibitors and Active Site Titrants for Cysteine Proteinases." J. Med. Chem. 41, 1344-1351 (1998).
- S. Liu and R. P. Hanzlik, "Effects of Ligand Homologation and Ligand Reactivity on the Apparent Kinetic Specificity of Papain." Biochim. Biophys. Acta, 1250, 43-48 (1995).
- K. L. Foje and R. P. Hanzlik, "Peptidyl Thioamides as Substrates and Inhibitors of Papain, and as Probes of the Kinetic Significance of the Oxyanion Hole." Biochim. Biophys. Acta, 1201, 447-453 (1994).
- S. Liu and R. P. Hanzlik, "Structure-Activity Relationships for Inhibition of Papain by Peptide Michael Acceptors." J. Med. Chem. 35, 1067-1075 (1992).
- R. P. Hanzlik, J. Zygmunt, and J. B. Moon, "Reversible Covalent Binding of Peptide Nitriles to Papain." Biochim. Biophys. Acta, 1035, 62-70 (1990).
- J. B. Moon and R. P. Hanzlik, "Enthalpy and Entropy of Hippuraldehyde Hydration and Binding to Papain." Biochim. Biophys. Acta, 914, 1-5 (1987).
- J. B. Moon, R. S. Coleman, and R. P. Hanzlik, "Reversible Covalent Inhibition of Papain by a Peptide Nitrile. 13C-NMR Evidence for a Thioimidate Ester Adduct." J. Am. Chem. Soc., 108, 1350-1351 (1986).
- S. A. Thompson, P. R. Andrews, and R. P. Hanzlik, "Carboxyl-modified Aminoacids and Peptides as Protease Inhibitors." J. Med. Chem. 29, 104-111, (1986).
- R. P. Hanzlik and S. A. Thompson, "Vinylogous Amino Acid Esters: A New Class of Inactivators for Thiol Proteases." J. Med. Chem., 27, 711-712, (1984).