Chemical Mechanisms of Cytotoxicity
The liver is a major site of metabolism for xenobiotic compounds, some of which are converted to chemically reactive metabolites capable of covalently modifying endogenous proteins and causing cytotoxicity. Understanding the mechanism(s) by which chemicals cause cytotoxic liver injury remains a major challenge of enormous scientific and practical importance. Hepatotoxicity is a major important reason for the failure of drug candidates late in development (when such failure is extremely expensive in terms of lost investment of resources), and in the withdrawal of approved drugs early from open clinical usage (when the cost of failure can also include severe or even fatal injury to patients). Examples of drugs that were withdrawn because of hepatotoxicity include troglitazone, tienilic acid, bromfenac and benoxaprofen. Even the common over-the-counter analgesic drug acetaminophen has significant hepatotoxic potential, and in the United States it is the single most common cause of acute liver failure!
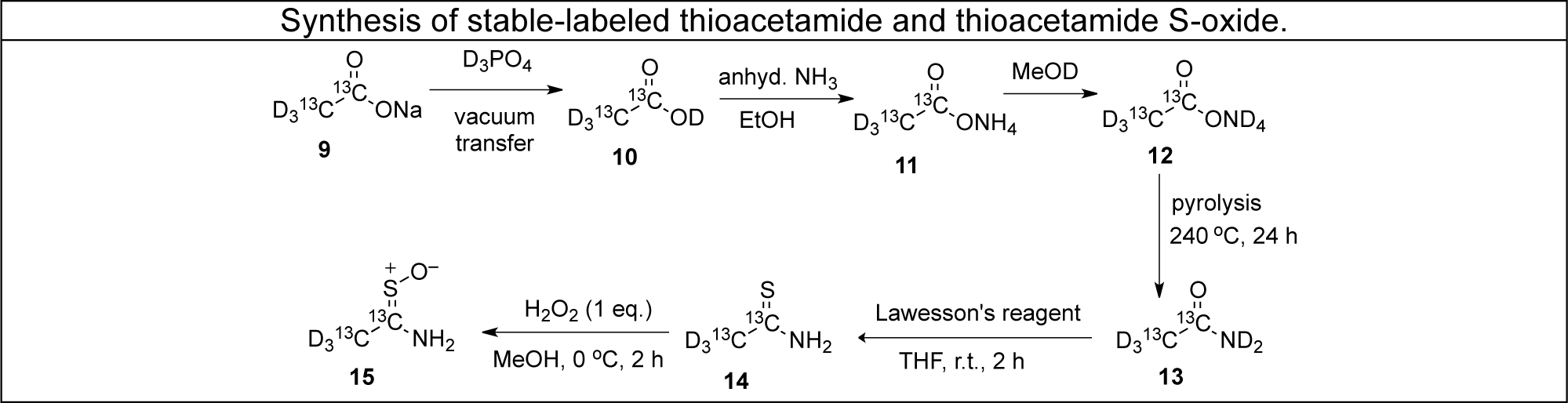
Our approach to elucidating mechanisms of toxicity involves the use of "model" hepatotoxic compounds such as bromobenzene, thioacetamide and thiobenzamide. We synthesize isotopic variants of these molecules and use them as probes of metabolism and covalent binding to proteins in vivo, in isolated hepatocytes and in enzymatic systems in vitro. Radioactive labeling with C-14 facilitates detection and quantitation, and stable-labeled forms with C-13 and /or deuterium facilitate mass spectral studies aimed at structure elucidation.
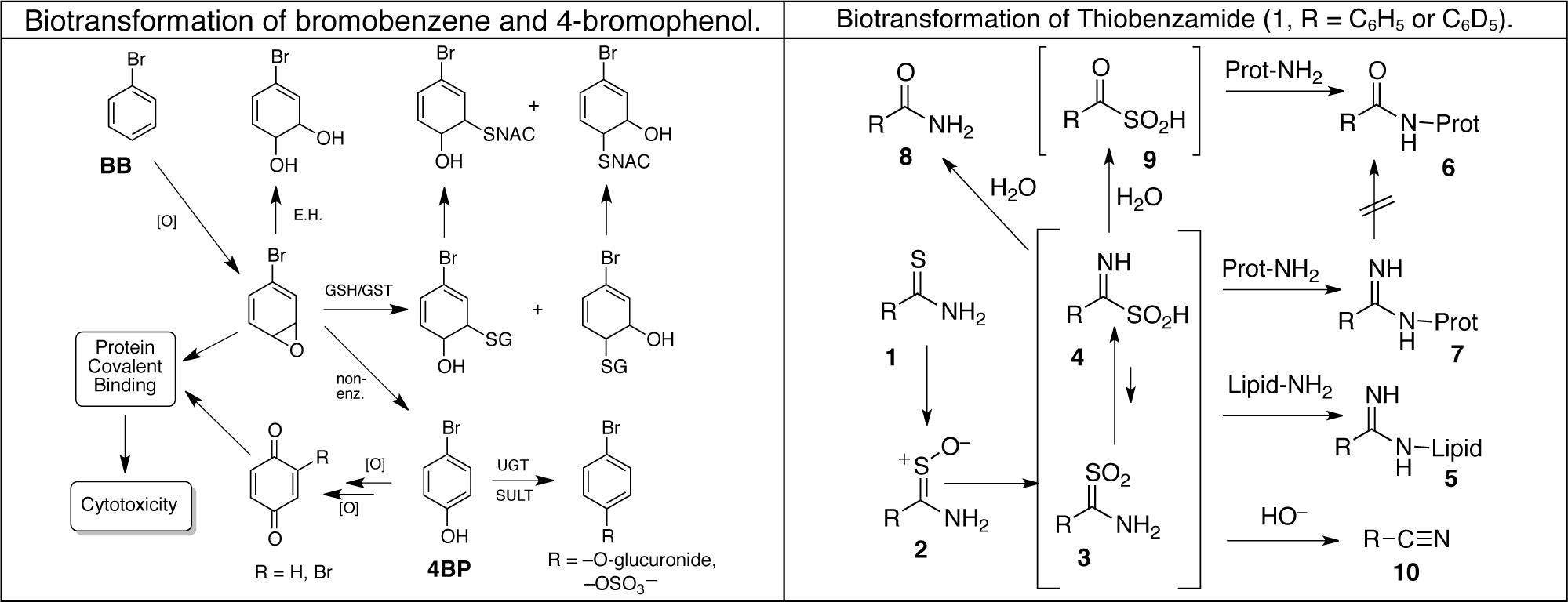 Using these techniques our laboratory has elucidated the molecular structures of numerous metabolites, protein adducts and even lipid adducts derived as shown above. We have used proteomic techniques and mass spectrometry to identify nearly 50 liver proteins targeted by reactive metabolites of bromobenzene in vivo. We have also identified over 60 liver protein targets of thiobenzamide metabolites and are currently working on thioacetamide covalent binding in isolated hepatocytes. The same techniques should allow us to utilize human-derived hepatocytes to assess whether animal studies actually do provide a good reflection of what goes on in human liver cells.
Using these techniques our laboratory has elucidated the molecular structures of numerous metabolites, protein adducts and even lipid adducts derived as shown above. We have used proteomic techniques and mass spectrometry to identify nearly 50 liver proteins targeted by reactive metabolites of bromobenzene in vivo. We have also identified over 60 liver protein targets of thiobenzamide metabolites and are currently working on thioacetamide covalent binding in isolated hepatocytes. The same techniques should allow us to utilize human-derived hepatocytes to assess whether animal studies actually do provide a good reflection of what goes on in human liver cells.
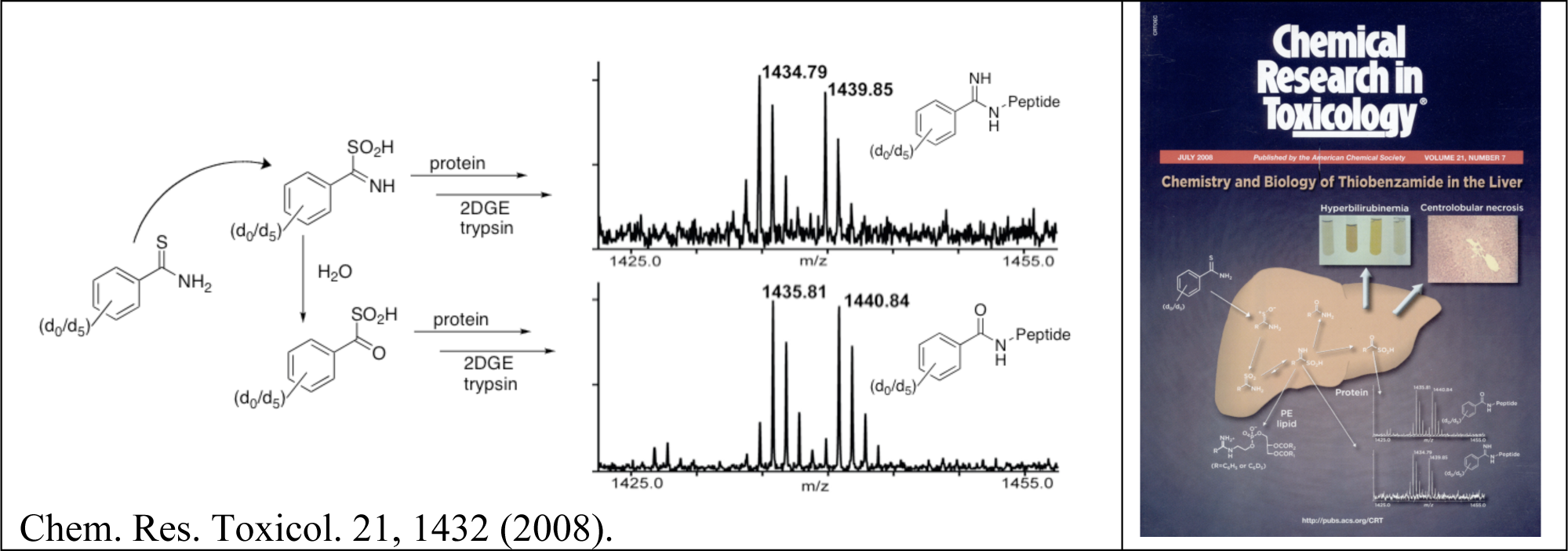 Another question we are pursuing is whether reactive metabolites of multiple compounds target more or less the same subset of liver proteins. We have built a large database of more than 300 proteins targeted by diverse reactive metabolites and this information is publicly available by request.
Another question we are pursuing is whether reactive metabolites of multiple compounds target more or less the same subset of liver proteins. We have built a large database of more than 300 proteins targeted by diverse reactive metabolites and this information is publicly available by request.
Finally, in collaboration with a bioinformaticist and a molecular pharmacologist, we are working to identify the interacting partners of reactive metabolite target proteins, and the signaling cascades triggered by adducted proteins that ultimately lead to cell death. Overall, this project is truly interdiscipinary in nature and in practice.
Publications
- Diganta Sarma and Robert P. Hanzlik, "Synthesis of carbon-14, carbon-13 and deuterium labeled thioacetamide and thioacetamide S-oxide" Journal of Labelled Compounds and Radiopharmaceuticals, (in press 2011).
- Jianwen Fang, Yakov Koen and Robert P. Hanzlik, "Bioinformatic analysis of xenobiotic reactive metabolite target proteins and their interacting partners." BMC Chemical Biology 2009, 9:5 (12 June 2009). PMID: 19523227
- M. A. Altaweel, J. VerHoeve, J. Eells, R. P. Hanzlik and B. Zhang, "Ocular and Systemic Safety Evaluation of Calcium Formate." J. Ocular Pharmacol. Toxicol. 25, 223-230 (2009). PMID: 19456257
- Robert P. Hanzlik, Jianwen Fang, Yakov M. Koen, "Filling and Mining the Reactive Metabolite Target Protein Database," Chem.-Biol. Interactions, 179, 38-44 (2009). PMID: 18823962
- Keisuke Ikehata, Tatyana G. Duzhak, Nadezhda A. Galeva, Tao Ji, Yakov M. Koen and Robert P. Hanzlik, "Protein Targets of Reactive Metabolites of Thiobenzamide in Rat Liver In Vivo." Chem. Res. Toxicol., 21, 1432-1442 (2008). PMID: 18547066
- Weijun Huang, Yasufumi Yamamoto, Yi Li, Dengfeng Dou, Kevin R. Alliston, R. P. Hanzlik and W. C. Groutas, "An X-ray Snapshot of the Mechanism of Inactivation of Human Neutrophil Elastase by 1,2,5-Thiazolidin-3-one 1,1-Dioxide Derivatives, J. Med. Chem. 51, 2003-2008 (2008). PMID: 18318470
- Robert P. Hanzlik, Yakov M. Koen, Bharga Theertham, Yinghua Dong and Jianwen Fang. "The reactive metabolite target protein database (TPDB) — a web-accessible resource." BMC Bioinformatics 8:95 (2007). doi: 10.1186/1471-2105-8-95. http://www.biomedcentral.com/1471-2105/8/95. PMID: 17367530
- T. Ji, K. Ikehata, Y.M. Koen, S.W. Esch, T.D. Williams and R.P. Hanzlik, "Covalent Modification of Microsomal Lipids by Thiobenzamides in Vivo." Chem. Res. Toxicol. 20, 701-708 (2007). PMID: 17381136
- Y.M. Koen, N.V. Gogichaeva, M.A. Alterman and R.P. Hanzlik, "A Proteomic Analysis of Bromobenzene Reactive Metabolite Targets in Rat Liver Cytosol." Chem. Res. Toxicol. 20, 511-519 (2007). PMID: 17305373
- Y.M. Koen, W. Yue, N.A. Galeva, T.D. Williams and R.P. Hanzlik, "Site-Specific Arylation of Rat Glutathione S-Transferase A1 and A2 by Bromobenzene Metabolites In Vivo." Chem. Res. Toxicol. 19, 1426-1434 (2006). PMID: 17112229
- Weimin Yue, Yakov M. Koen, Todd D. Williams, and Robert P. Hanzlik, "Use of Isotopic Signatures for Mass Spectral Detection of Protein Adduction by Chemically-Reactive Metabolites of Bromobenzene. Studies with Model Proteins." Chem. Res. Toxicol., 18, 1748-1754 (2005).
- Weimin Yue, S. I. Lewis, Y. M. Koen, and R. P. Hanzlik, "Synthesis of N(t)-Arylhistidine Derivatives via Direct N-Arylation," Bioorg. Med. Chem. Let., 14, 1637-1640 (2004).
- Yakov M. Koen and Robert P. Hanzlik, " Identification of Seven Proteins in the Endoplasmic Reticulum as Targets for Reactive Metabolites of Bromobenzene." Chem. Res. Toxicol., 15, 699-706 (2002).
- Y. M. Koen, T. D. Williams, and R. P. Hanzlik, "Identification of Three Protein Targets for Reactive Metabolites of Bromobenzene in Rat Liver Cytosol." Chem. Res. Toxicol., 13, 1326-1335 (2000).
- E. M. Rombach and R. P. Hanzlik, "Detection of Adducts of Bromobenzene-2,3-oxide to Rat Liver Microsomal Protein Sulfhydryl Groups Using Specific Antibodies." Chem. Res. Toxicol., 12, 159-163 (1999).
- E. M. Rombach and R. P. Hanzlik, "Identification of a Rat Liver Microsomal Esterase as a Target Protein for Bromobenzene Metabolites." Chem. Res. Toxicol., 11, 178-184 (1998).
- M. C. M. Reitjens, C. den Besten, R. P. Hanzlik, and P. J. van Bladeren, "The Cytochrome P450 Catalyzed Oxidation of Halobenzene Derivatives." Chem. Res. Toxicol., 10, 629-635 (1997).
- E. M. Rombach and R. P. Hanzlik, "Detection of Benzoquinone Adducts to Rat Liver Protein Sulfhydryl Groups Using Specific Antibodies." Chem. Res. Toxicol., 10, 1407-1411 (1997).
- R. B. Bambal and R. P. Hanzlik, "Bromobenzene-3,4-oxide Alkylates Histidine and Lysine Side Chains of Rat Liver Protein in vivo." Chem. Res. Toxicol., 8, 729-735 (1995).
- R. P. Hanzlik, S. P. Harriman, and M. Frauenhoff, "Covalent Binding of Benzoquinone to Reduced Ribonuclease. Adduct Structures and Stoichiometry." Chem. Res. Toxicol., 7, 177-184 (1994).
- R. Bambal and R. P. Hanzlik, "Covalent Addition of Bromobenzene-3,4-oxide to Protein. Synthesis of N(epsilon)-(p-bromophenyl)-Lysine and N(tau)-(p-Bromophenyl)-L-Histidine as Model Adducts." J. Org. Chem., 59, 729-732 (1994).