Drug Metabolism: Enzyme Mechanisms and Inhibition
Many potential drug candidates ultimately fail in practice because they are metabolized too efficiently as they are being absorbed. Often the problem-causing biotransformation is an oxidative N-dealkylation reaction catalyzed by a cytochrome P450 enzyme. Some years ago, as a means of overcoming this "first-pass effect," we attempted to design compounds that might inhibit the P450s involved. We found that simple cyclopropylamines, such as N-benzylcyclopropylamines (BCA) were extremely effective as P450 inhibitors, and later showed that they were actually suicide substrates for P450 enzymes.
To explain this unique activity of cyclopropylamines we proposed that in addition to abstracting hydrogen atoms (Scheme 1) or donating oxygen atoms to its substrates, P450 enzymes could also oxidize certain substrates by a single electron transfer (Scheme 2).
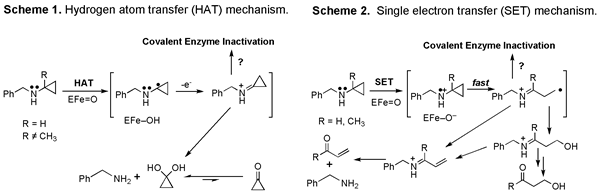
Adding a methyl group (R) to the cyclopropane ring of BCA in Scheme 1 blocked N-dealkylation but not suicide substrate activity. Thus Scheme 2 became attractive to account for both the N-delakylation of ordinary amines and the suicide substrate activity unique to cyclopropylamines. Over time the cyclopropyl group became widely adopted as a mechanistic probe for amine metabolzing enzymes and chemical model systems, and Scheme 2 became a widely accepted mechanism for amine metabolism.
Unfortunately, while Scheme 2 garnered much support from chemical model systems, comparable evidence for its involvement in enzymatic oxidation was not forthcoming. Therefore we returned to this problem several years ago. In particular, we wanted to obtain definitive evidence for the fate of the cyclopropyl carbons that are removed during N-dealkylation of cyclopropylamines. We anticipated that formation of cyclic vs. ring-opened products would distinguish between HAT and SET mechanisms.
Using horseradish peroxidase (HRP) as a model amine-oxidizing enzyme and N-cyclopropyl-N-methylaniline (NCNMA) as a substrate, we demonstrated that SET oxidation of NCNMA led exclusively to ring opening of the cyclopropyl group, followed in this case by an intramolecular reaction with the phenyl moiety to produce a quinoline derivative.
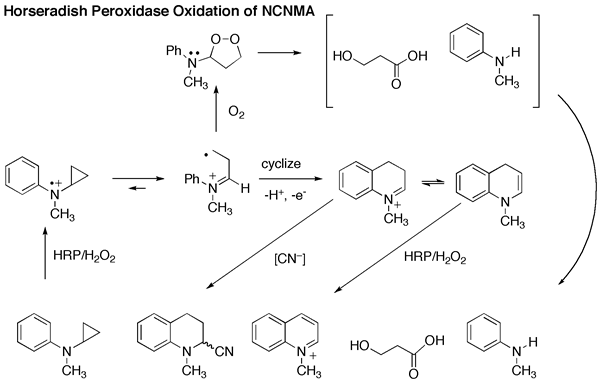
However, when we submitted NCNMA to P450 oxidation, not only was there no ring opening, there was also no suicide enzyme inactivation! Instead, the only products were formaldehyde and cyclopropanaone hydrate. Clearly, P450 uses a HAT mechanism (Scheme 1) in this case.
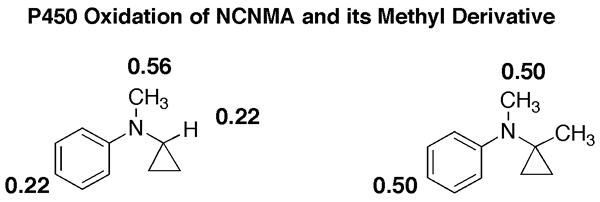
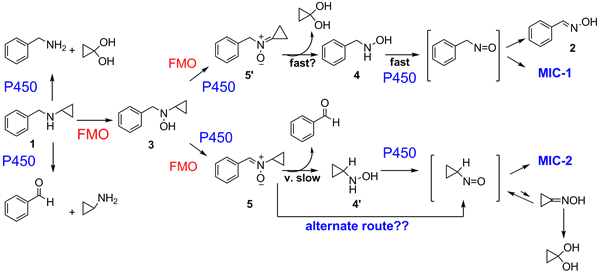
We then returned to investigate BCA-P450 interactions more closely, and got another big suprise. BCA is oxidized to a nitroso compound that complexes the heme iron of P450 extremely tight thereby inactivating the enzyme! However, the amount of complex formed is less than the amount of the enzyme inactivated, so additional mechanisms for enzyme inactivation must exist. We are presently trying to determine if Scheme 2 is one of them.
Publications
- M. A. Cerny and R. P. Hanzlik, "Cytochrome P450-Catalyzed Oxidation of N-Benzyl-N-cyclopropylamine Generates both Cyclopropanone Hydrate and 3-Hydroxypropionaldehyde via Hydrogen Abstraction, not Single Electron Transfer." J. Am. Chem. Soc., 128, 3346-3354 (2006).
- M. A. Cerny and R. P. Hanzlik, "Cyclopropylamine inactivation of cytochromes P450. Role of metabolic intermediate complexes." Arch. Biochem. Biophys., 436, 265-275 (2005).
- R. P. Hanzlik, S. P. Harriman, C. L. Shaffer, Y. M. Koen, R. A. Totah, and M. A. Cerny, "The Oxidative Metabolism of Cyclopropylamines: Fate of the Three Carbons and Other Interesting Observations." Synthesis and Applications of Isotopically Labelled Compounds, 8, 111-114 (2004).
- R. A. Totah and R. P. Hanzlik, "Non-Oxidative Decarboxylation of Glycine Derivatives by a Peroxidase." J. Am. Chem. Soc., 124, 10000-10001 (2002).
- C. L. Shaffer, Yakov M. Koen, and R. P. Hanzlik, "Formation of Cyclopropanone during Cytochrome P450-Catalyzed N-Dealkylation of a Cyclopropylamine." J. Am Chem. Soc., 124, 8268-8274 (2002).
- R. A. Totah and R. P. Hanzlik, "Detection of Aminium Ion Intermediates: N-cyclopropyl vs. N-Carboxymethy Groups as Reporters." J. Am. Chem. Soc., 123, 10107-10108 (2001).
- C. L. Shaffer, M. D. Morton, and R. P. Hanzlik, "Enzymatic N-Dealkylation of an N-Cyclopropylamine. Fate of the Cyclopropyl Group." J. Am Chem. Soc., 123, 8502-8508 (2001).
- C. L. Shaffer, M. D. Morton, and R. P. Hanzlik, "Enzymatic N-Dealkylation of an N-Cyclopropylamine: An Unusual Fate for the Cyclopropyl Group." J. Am Chem. Soc., 123, 349-350 (2001).
- H. Chen, M. deGroot, N. P. E. Vermeulen, and R. P. Hanzlik, "Oxidative N-Dealkylation of p-Cyclopropyl-N,N-dimethylaniline. An Extreme Substituent Effect on a Radical-Clock Reaction Explained by ab initio Calculations on Radical Cation Intermediates." J. Org. Chem., 62, 8227-8230 (1997).