Research Overview—
Research areas include asymmetric total synthesis of natural and non-natural compounds of biomedical significance (anti-infectives and anti-cancer), and, development of new organic synthetic methodologies. An important tool in our synthetic endeavors have been the utilization of easily available enantiopure amino acids as chiral building blocks towards stereoselective synthesis of desired target compounds (chiral pool approach).
The synthetic efforts included development of compounds targeting:
- HIV Reverse Transcriptase (AIDS)
- Chitin Synthase (anti-fungal)
- Endotoxins / Lipopolysachharides (septic shock)
- Proteasome (anti-cancer)
Some of the natural product targets in our total synthesis studies include:
- The potent antifungal complex nucleoside antibiotics, Polyoxins, Nikkomycins, Ezomycins, Amipurimycin etc.
- Proteasome inhibitor novel cytotoxic marine natural product Salinosporamide A.
Additionally, employing combinatorial chemistry (CombiChem) approaches, synthesis of libraries of biomimetics (novel nucleosides, azacarbohydrates etc) and their subsequent high throughput screening (HTS) against various biological targets have also been the target of our research.
Lab Members —
Selected Publications —
- Intramolecular azide-alkyne [3+2] cycloaddition: Versatile route to new heterocyclic structural scaffolds. Li, R.; Jansen, D. J.; Datta, A. Org. Biomol. Chem. 2009, 7, 1921-1930.
- Studies on peptidyl nucleoside antibiotics: Synthesis and antifungal evaluation of pyranosyl nucleoside analogs of nikkomycin. C. S. Stauffer, A. W. Fothergill, M. G. Rinaldi, and A. Datta. Future Med. Chem. 2009, 1, 379-389.
- Synthetic studies on ezomycins: Stereoselective route to a thymine octosyl nucleoside derivative. J. K. Khalaf, David G. VanderVelde, and A. Datta. J. Org. Chem. 2008, 73, 5977-5984.
- Controlling plasma protein binding: Structural correlates of interactions of hydrophobic polyamine endotoxin sequestrants with human serum albumin. T. B. Nguyen, E. V. K. Suresh Kumar, D. Sil, S. J. Wood, K. A. Miller, H. J. Warshakoon, A. Datta, S. A. David. Mol. Pharm. 2008, 5, 1131-1137.
- A rapid synthetic route to conformationally restricted [5,5]-bicyclic nucleoside-amino acid conjugates. K. W. C. Poon, A. Datta. Nucleosides, Nucleotides & Nucleic Acids. 2008, 27, 914-930.
- Pharmacokinetics of DS-96, an alkylpolyamine lipopolysaccharide sequestrant, in rodents. R. Li, A. Shrestha, D. Sil, N. H. Pardeshi, N. Schwarting, K. S. Schrono, R. A. Rajewski, A. Datta, S. A. David. J. Pharm. Sci. 2008, 97, 5376-5385.
- A concise, asymmetric synthesis of (2R,3R)-3-hydroxyaspartic acid. J. K. Khalaf, A. Datta. Amino Acids 2008, 35, 507-510.
- N-Methylation of the C3 amide of taxanes: Synthesis of N-methyltaxol C and N-methylpaclitaxel. H. K. R. Santhapuram, O. E. Hutt, A. Datta, G. I. Georg. J. Org. Chem. 2008, 73, 4705-4708.
- Synthetic studies on amipurimycin: Total synthesis of a thymine nucleoside analog. C. S. Stauffer, A. Datta. J. Org. Chem. 2008, 73, 4166-4174.
- De novo synthetic route to a combinatorial library of peptidyl nucleosides. K. W. C. Poon, N. Liang, A. Datta. Nucleosides, Nucleotides & Nucleic Acids. 2008, 27, 389-407.
- Trimethylsilyl trifluoromethanesulfonate (TMSOTf) assisted facile deprotection of N,O-acetonides. K. W. C. Poon, K. M. Lovell, K. N. Dresner, A. Datta. J. Org. Chem. 2008, 73, 752-755.
- Total synthesis and antifungal activity of a carbohydrate ring-expanded pyranosyl nucleoside analog of nikkomycin B. C. S. Stauffer, P. Bhaket, A. W. Fothergill, M. G. Rinaldi, A. Datta. J. Org. Chem. 2007, 72, 9991-9997.
- Structure-based quantitative structure activity relationship analysis of Omuralide analogs in the 20S proteasome: A covalent Inhibitor COMBINE Study. J. L. Wang, A. Datta, G. H. Lushington. Lett. Drug Design 2007, 4, 417-421.
- Bound to shock: Protection from lethal endotoxemic shock by a novel, nontoxic, alkylpolyamine lipopolysaccharide sequestrant. D. Sil, A. Shrestha, M. R. Kimbrell, T. B. Nguyen, A. K. Adisechan, R. Balakrishna, B. G. Abbo, S. Malladi, K. A. Miller, S. Short, J. R. Cromer, S. Arora, A. Datta, S. A. David. Antimicrob. Agents Chemother. 2007, 51, 2811-2819.
- Protection from endotoxic shock by EVK-203, a novel alkylpolyamine sequestrant of lipopolysaccharide. T. B. Nguyen, A. K. Adisechan, E. V. K. Suresh Kumar, R. Balakrishna, M. R. Kimbrell, K. A. Miller, A. Datta, S. A. David. Biorg. Med. Chem. 2007, 15, 5694-5709.
- Oxidation of baccatin III at C14: A facile rearrangement of the baccatin III core. D. Dutta, A. Datta, D. G. Vander Velde, G. I. Georg. Lett. Org. Chem. 2007, 4, 151-154.
- From dioxin to drug lead - The development of 2,3,7,8-tetrachlorophenothiazine. K. W. Fried, C. M. Schneider, K-W. Schramm, A. Datta, N. Chahbane, C. Corsten, D. R. Powell, D. Lenoir, A. Kettrup, P. Terranova, G. I. Georg, K. K. Rozman. ChemMedChem, 2007, 2, 890-897.
- Structural correlates of antibacterial and membrane-permeabilizing activities in acylpolyamines. Rajalakshmi Balakrishna, S. J. Wood, T. B. Nguyen, K. A. Miller, E.V.K. Suresh Kumar, A. Datta, S. A. David: Antimicrob. Agents Chemother. 2006, 50, 852-861.
- Single-site chemical modification at C10 of the baccatin III core of paclitaxel and taxol C reduces P-glycoprotein interactions in bovine brain microvessel endothelial cells. J. T. Spletstoser, B. J. Turunen, K. Dessino, A. Rice, A. Datta, D. Dutta, J. K. Huff, R. H. Himes, K. L. Audus, A. Seelig and G. I. Georg : Bioorg. Med. Chem. Lett. 2006, 16, 495-498.
- Stereoselective total synthesis of cis- and trans-3-hydroxypipecolic acids. N. Liang, A. Datta. J. Org. Chem. 2005, 70, 10182-10185.
- A Stereoselective route to cis-2-phenyl-3-piperidinol. N. Liang, P. Srinivas, A. Datta. Tetrahedron Lett. 2005, 46, 7221-7223.
- Stereoselective synthesis of a novel carbamoyl oxybiotin. C. S. Stauffer, A. Datta. Tetrahedron Lett. 2005, 46, 6469-6471.
- Stereoselective Route to the Ezoaminuroic Acid Core of the Ezomycins. J. K. Khalaf, A. Datta. J. Org. Chem. 2005, 70, 6937-6940.
- Lipopolysaccharide Sequestrants: Structural Correlates of Activity and Toxicity in Novel Acylhomospermines. K. A. Miller, E. V. K. Suresh Kumar, S. J. Wood, J. R. Cromer, A. Datta, S. A. David. J. Med. Chem. 2005, 48, 2589-2599.
- Total Synthesis of Cytotoxic Anhydrophytosphingosine Pachastrissamine (Jaspine B). P. Bhaket, K. Morris, C. S. Stauffer, A. Datta. Org. Lett. 2005, 7, 875-876.
- Complex Peptidyl Nucleoside Antibiotics: Efficient Syntheses of the Glycosyl Nucleoside Amino Acid Cores. P. Bhaket, C.S. Stauffer, A. Datta. J. Org. Chem. 2004, 69, 8594-8601.
- An Efficient and Highly Stereocontrolled Route to Bulgecinine Hydrochloride. J. K. Khalaf, A. Datta. J. Org. Chem. 2004, 69, 387-390.
- Efficient Synthesis of (S,S)-Ethambutol from L-Methionine. C.S. Stauffer, A. Datta. Tetrahedron 2002, 58, 9765-9767.
- Efficient Synthesis of the 3'-Phenolic Metabolite of Paclitaxel. S. H. K. Reddy, A. Datta, G. I. Georg. J. Org. Chem. 2001, 66, 8211-8214.
- Novel D-seco paclitaxel analogs: Synthesis, biological evaluation, and model testing. L. Barboni, A. Datta, D. Dutta, G. I. Georg, D. G. Vander Velde, R. H. Himes, M. Wang, J. P. Snyder. J. Org. Chem. 2001, 66, 3321-3329.
- Stereoselective Total Synthesis of (-)-Deoxoprosophylline. A. Datta, J. S. Ravi Kumar, S. Roy. Tetrahedron 2001, 57, 1169-1173.
- A Stereoselective Route to Hydroxyethylamine Dipeptide Isosteres. A. Datta, G. Veeresa. J. Org. Chem. 2000, 65, 7609-7611.
- Stereoselective total synthesis of (+)-Azimic acid. K. Kiran Kumar, A. Datta. Tetrahedron 1999, 55, 13899-13906.
- Di-tert-Butyl Dicarbonate: A Novel Reagent for the Efficient Synthesis of Dipeptides Under Mild Conditions. D. K. Mohapatra, A. Datta. J. Org. Chem. 1999, 64, 6879-6880.
- Stereoselective Synthesis of Novel Cyclopropyl Analogs of Known Cysteine Protease Inhibitors. J. S. Ravi Kumar, S. Roy, A. Datta. Bioorg. Med. Chem. Lett. 1999, 9, 513-514.
- Stereoselective Synthesis of N-Boc Galantic Acid Ethyl Ester. J. S. Ravi Kumar, A. Datta. Tetrahedron Lett. 1999, 40, 1381-1384.
- Stereoselective Synthesis of Antifungal Antibiotic (+)-Preussin. G. Veeresa, A. Datta. Tetrahedron 1998, 54, 15673-15678.
- The Chemistry of Taxane Diterpene: Stereoselective Reductions of Taxanes. G. I. Georg, G. C. B. Harriman, A. Datta, S. Ali, Z. Cheruvallath, D. Dutta, D. G. Vander Velde and R. H. Himes. J. Org. Chem. 1998, 63, 8926-8934.
- Stereoselective Synthesis of Chloramphenicol from D-Serine. G. Veeresa, A. Datta. Tetrahedron Lett. 1998, 39, 8503-8504.
- Stereoselective Synthesis of the C1-C10 Fragment of Constanolactones A and B. S. Varadarajan, D. K. Mohapatra, A. Datta. Tetrahedron Lett. 1998, 39, 5667-5670.
- Stereoselective Synthesis of (2S,3R)-N-Boc-3-Hydroxyglutamic Acid. G. Veeresa., A. Datta. Tetrahedron Lett. 1998, 39, 3069-3070.
- Studies towards the total syntheses of solandelactones : Stereoselective synthesis of the cyclopropane-lactone segment. S. Varadarajan, D. K. Mohapatra, A. Datta. Tetrahedron Lett. 1998, 39, 1075-1078.
- Stereoselective Synthesis of a Key Precursor of Halicholactone and Neohalicholactone. D. K. Mohapatra, A. Datta. J. Org. Chem. 1998, 63, 642-646.
- Stereoselective Synthesis of Polyoxamic Acid from (R)-Phenylglycine. G. Veeresha, A. Datta. Tetrahedron Lett. 1998, 39, 119-122.
- Efficient Syntheses of Biologically Active Chiral 2-Alkylamino Benzoxazinones. D. K. Mohapatra, A. Datta. Bioorg. Med. Chem. Lett. 1997, 7, 2527-2530.
- Efficient Conversion of (S)-Methionine into (R)-Garner Aldehyde. J. S. Ravi Kumar, A. Datta. Tetrahedron Lett. 1997, 38, 6779-6780.
- Stereoselective Synthesis of (-)-N-Boc-Statine and (-)-N-Boc-Norstatine. G. Veeresha, A. Datta. Tetrahedron Lett. 1997, 38, 5223-5224.
- First Syntheses of (2S,3S)- and (2S,3R)-m-Prenyl-β-Hydroxytyrosine Derivatives. Bioactive Amino Acid Fragment of a Substance P Antagonist Novel Cyclic Peptide. J. S. Ravi Kumar, A. Datta. Tetrahedron Lett. 1997, 38, 473-476.
- Di-tert-Butyl Pyrocarbonate Mediated Cyclodehydration of N-Acyl Amino Acids into Functionalized Oxazoles and Acylanthranils. D. K. Mohapatra, A. Datta. Synlett 1996, 1129-1130.
- Syntheses of Novel C-9 and C-10 Modified Bioactive Taxanes. A. Datta, D. G. Vander Velde, G. I. Georg, R. H. Himes. Tetrahedron Lett. 1995, 36, 1985-1988.
- Synthesis of Biologically Active 2-Benzoyl Paclitaxel Analogs. G. I. Georg, S. M. Ali, T. C. Boge, A. Datta, L. Falborg, H. Park, M. Mejillano, R. H. Himes. Bioorg. Med. Chem. Lett. 1995, 5, 259-262.
- Selective Deesterification Studies on Taxanes: Simple and Efficient Hydrazinolysis of C-10 and C-13 Ester Functionalities. A. Datta, M. Hepperle, G. I. Georg. J. Org. Chem. 1995, 60, 761-763.
- Synthesis of 2-O-Heteroaroyl Taxanes: Evaluation of Microtubule Assembly Promotion and Cytotoxicity. G. I. Georg, G. C. B. Harriman, S. M. Ali, A. Datta, M. Hepperle, R. H. Himes. Bioorg. Med. Chem. Lett. 1995, 5, 115-118.
- Selective C-2 and C-4 Deacylation and Acylation of Taxol: The First Synthesis of a C-4 Substituted Taxol Analogue. G. I. Georg, S. M. Ali, T. C. Boge, A. Datta, L. Falborg, R. H. Himes. Tetrahedron Lett. 1994, 35, 8931-8934.
- 4-Deacetyltaxol and 10-Acetyl-4-Deacetyltaxotére : Synthesis and Biological Evaluation. A. Datta, L. R. Jayasinghe, G. I. Georg. J. Med. Chem. 1994, 37, 4258-4260.
- Internal Nucleophile Assisted Selective Deesterification Studies on Baccatin III. Synthesis of 2-Debenzoyl and 4-Deacetyl Baccatin III Analogs. A. Datta, L. R. Jayasinghe, G. I. Georg. J. Org. Chem. 1994, 59, 4689-4690.
- The First Synthesis of a C-9 Carbonyl Modified Baccatin III and Its Conversion to Novel Taxol and Taxotére Analogs. A. Datta, J. Aubé, G. I. Georg, L. A. Mitscher, L. R. Jayasinghe. Bioorg. Med. Chem. Lett. 1994, 4, 1831-1834.
- Structure-Activity Studies of Antitumor Taxanes: Synthesis of Novel C-13 Side-Chain Homologated Taxol and Taxotére Analogs. A. Datta, S. M. Ali, J. Zygmunt, D. G. Vander Velde, G. I. Georg, L. R. Jayasinghe. J. Med. Chem. 1994, 37, 2981-2984.
- Chiral β-C-lithiated β-alkoxy acrylates: Efficient synthons for highly functionalized cyclopentenones. A. Datta, R. R. Schmidt. Tetrahedron Lett. 1993, 34, 4161-4164.
- A convenient stereoselective synthesis of a (-)-Vertinolide precursor. A. Datta, D. Dutta, R. R. Schmidt. Tetrahedron Lett. 1992, 33, 8035-8038.
- A regioselective synthesis of 5-substituted 3-methyl tetronic acid derivatives. A. Datta, R. R. Schmidt. Synlett 1992, 429-430.
- Reformatsky reaction on α-oxoketene dithioacetals; Synthesis of substituted and fused ethyl-2-hydroxy-6-methylthiobenzoates, 6-methylthio-3(1H)pyridones and 6-methyl-thiopyran-2-one derivatives. A. Datta, H. Ila, H. Junjappa. J. Org. Chem. 1990, 55, 5589-5594.
- Darzen's reaction on oxoketene dithioacetals; A versatile synthesis of substituted and annelated furan-2-carboxylates. A. Datta, D. Pooranchand, H. Ila, H. Junjappa. Tetrahedron 1989, 23, 7631.
- Oxymercuration-reduction of 3-bis(methylthio)methylene-ϒ,δ-unsaturated alcohols; Synthesis of 3-bis(methylthio)methylene-2,5-substituted tetrahydrofurans. A. Datta, S. Bhattacharjee, H. Ila, and H, Junjappa. Synthesis 1988, 725-727.
- A novel route to methyl 3-(3,4-disubstituted 5-alkylthio / amino-2-thienyl)propenoates. A. Datta, H. Ila, H. Junjappa. Synthesis 1988, 556-557.
- Novel cycloaromatization in Reformatsky reaction on α-oxoketene dithioacetals ; A regioselective synthesis of substituted ethyl-2-hydroxy-6-methylthiobenzoates. A. Datta, H. Ila, H. Junjappa. Tetrahedron Lett. 1988, 29, 497-500.
- Reformatsky reaction on aroylketene S,N-acetals; A facile route to 4-amino-6-aryl 2H-pyran-2-one. A. Datta, H. Ila, H. Junjappa. Synthesis 1988, 248-250.
- A stereoselective synthesis of α-ylidene-ϒ-butyrolactones. A. Datta, H. Ila, H. Junjappa. Tetrahedron 1987, 43, 5367-5374.
- Reformatsky reaction on α-oxoketene S,S-acetals: Synthesis of novel polarized vinylketene S,S-acetals and dimethyl-2-aryl / methyl-propen -1,3-dicarboxylates. S. Apparao, A. Datta, H. Ila, H. Junjappa. Synthesis 1985, 169-171.
- Polarized ketene dithioacetals. Studies on base catalysed rearrangements of 2-Bis(methylthio)methyleneindan-1-one, 2-Bis(methylthio)methylene-1-tetralone and 3-Bis (methylthio)methylene 2,3-dihydro-1-benzothio-pyran-4-one. S. Apparao, A. Datta, H. Ila, H. Junjappa. J.C.S. Perkin Trans I. 1984, 921-924.
Book Chapters and Reviews
- L. A. Mitscher, A. Dutta. "Antitumor Natural Products" in Drug Discovery and Development, Vol. 1: Drug Discovery. Mukund S. Chorghade (Ed.), John Wiley & Sons, New York. 2006, pp. 103-128.
- L. A. Mitscher, A. Dutta. "Contemporary Drug Discovery" in Burger's Medicinal Chemistry and Drug Discovery. Donald J. Abraham (Ed.), John Wiley & Sons, New York, 6th edition, Vol. 5 (Chemotherapeutic Agents), 2003, pp. 107-150.
- L. A. Mitscher, A. Dutta. "Combinatorial Chemistry and Multiple Parallel Synthesis" in Burger's Medicinal Chemistry and Drug Discovery. Donald J. Abraham (Ed.), John Wiley & Sons, New York, 6th edition, Vol. 2 (Drug Development), 2003, pp. 1-36.
- The Medicinal Chemistry of Taxol: Chemistry, Structure-Activity Relationships and Conformational Analysis. G. I. Georg, G. C. B. Harriman, D. G. Vander Velde, T. C. Boge, Z. S. Cheruvallath, A. Datta, M. Hepperle, H. Park, R. H. Himes and L. R. Jayasinghe In Taxane Anti-cancer Agents: Basic Science and Current Status. Georg G. I., Chen T. C., Ojima I.,Vyas, D. M. Eds.; American Chemical Society, Washington, DC, 1995; Chapter 16, pp 217-232.
- Novel Asymmetric Synthesis of β-Lactams with 3-(1'-Hydroxyethyl) Substituents: Boron reagent Mediated Aldol Reactions of Chiral Imines and Silyl Ketene Acetals Derived from 3-Hydroxybutyrate. A. Datta, G. I. Georg. Chemtracts 1994, 7, 306-310.
Patent
- K. K. Rozman; K. W. Fried; P. F. Terranova; G. I. Georg; A. Dutta. Phenothiazine Derivatives and Their Method of Use. United States Patent Application 10/875,864; PCT Patent Application PCT/US05/025131 2005.
Projects, Grants and Awards —
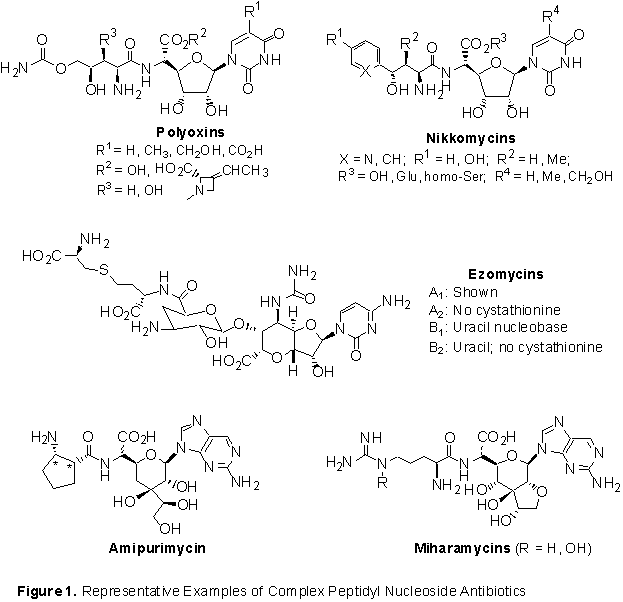
Complex Peptidyl Nucleoside Antibiotics as Novel Antifungal Agents
Streptomyces-derived complex peptidyl nucleoside antibiotics (Figure 1) represent a unique class of natural products with potent antifungal activity against various pathogenic fungi. The demonstrated antifungal activity, fungal cell specificity, potentially novel mechanism of action, and unique structural features of these peptidyl nucleosides have generated considerable interest in their potential utility as new generations of antifungal drugs. To better understand the structural requirements for optimum activity of the antifungal peptidyl nucleosides, the objective of our present research is to develop efficient synthetic routes to these natural products and further application of the methods towards design and synthesis of analogs with more potent activity and improved fungal cell permeability.

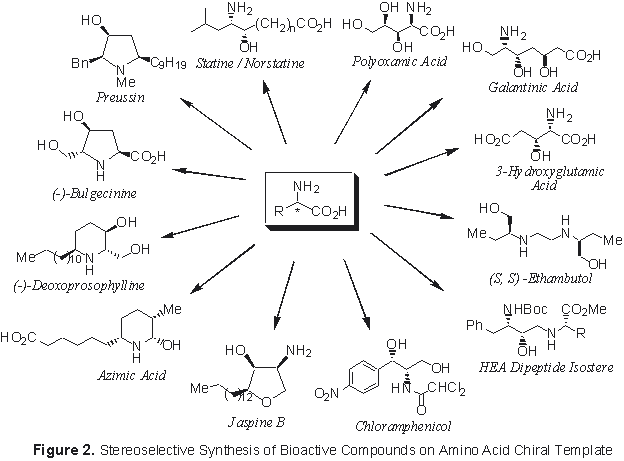
Amino Acid Chiral Template Assisted Stereoselective Synthesis of Bioactive Compounds
Synthesis of bioactive compounds in enantiopure form, utilizing amino acids as chiral template, has been the major goal of this project. Among the various molecules synthesized so far (Figure 2) are the non-proteinogenic hydroxy amino acids statine, polyoxamic acid, galantinic acid, and 3-hydroxyglutamic acid, the potent antibiotics chloramphenicol, bulgecinine, and preussin, and the all syn-tri substituted piperidine core of the plant alkaloids carpaine and azimine. A novel synthetic route to potent HIV protease inhibitor hydroxyethylamine (HEA) dipeptide isosteres has also been developed.

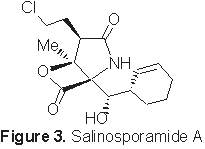
Salinosporamide A: A Proteasome Inhibitor, Cytotoxic Natural Product
The direct relevance of proteasome function in neoplastic processes has resulted in a great deal of interest in exploring the possibility that suitable proteasome inhibitors may prove useful as novel cancer therapeutic agents. Salinosporamide A (Figure 3), a recently isolated a marine bacterium metabolite has showed impressive proteasome inhibition and cytotoxic activity. The present research is focused on the total synthesis, detailed structure-activity relationship investigation and development of more potent analogs based on the natural product lead.
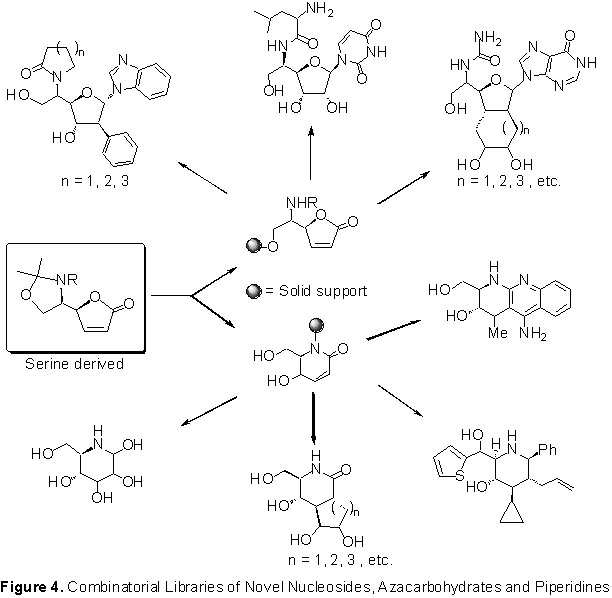
Combinatorial Libraries and Methodology Development (CMLD): Biomimetics
The main objective of this project is the development of new strategies and approaches for preparing combinatorial libraries of biomimetics � compounds that mimic the building blocks of life (e.g. amino acids, carbohydrates, nucleosides etc.). The research focus is on: (i) investigation of stereocontrolled carbon-carbon and carbon-heteroatom bond forming reactions on a solid support bound chiral template, and, (ii) application of these methods for the combinatorial synthesis of novel nucleosides, azacarbohydrates, and polyfunctionalized pipieridine scaffolds (Figure 4).